**Pharmacology and Pharmacokinetics of ∆-8-THC**:
– ∆-8-THC acts as a partial agonist of CB1 and CB2 cannabinoid receptors.
– It is moderately less potent than ∆-9-THC and has a lower binding efficiency to cannabinoid receptors.
– Following ingestion, ∆-8-THC undergoes conversion into metabolites like 11-hydroxy-∆-THC before excretion.
– The pharmacokinetic profile of ∆-8-THC is similar to ∆-9-THC.
– ∆-8-THC is a tricyclic terpenoid with distinct physical and chemical properties, including increased stability due to structural differences.
**Safety and Toxicity Concerns of ∆-8-THC**:
– The safety profile of long-term ∆-8-THC use is unknown, with reported adverse events and fatalities.
– US poison control centers received numerous exposure cases related to ∆-8-THC products.
– Concerns exist regarding unknown risks of psychosis, addiction, and regulatory oversight.
– Calls for further research and clinical caution regarding the potential toxic effects of ∆-8-THC.
**Medical Uses and Effects of ∆-8-THC**:
– ∆-8-THC exhibits antiemetic, anxiolytic, analgesic, appetite-stimulating, and neuroprotective properties.
– Studied applications include pediatric oncology for antiemetic effects and potential neuroinflammatory conditions.
– Research suggests a range of medical uses for ∆-8-THC, emphasizing its therapeutic potential in various conditions.
**Regulation and Legal Issues Surrounding ∆-8-THC**:
– ∆-8-THC faces varied legal status across jurisdictions, with regulatory bodies monitoring products.
– Instances of legal actions, arrests, and challenges regarding the sale of ∆-8-THC products have been reported.
– Concerns about the lack of oversight, quality control, and public health implications have prompted regulatory responses.
– The FDA has issued warnings and taken action against businesses marketing ∆-8-THC for therapeutic use.
**Research, Market Trends, and Access to ∆-8-THC**:
– Limited large clinical studies on ∆-8-THC exist, with ongoing research on its pharmacologic and pharmacokinetic similarities with ∆-9-THC.
– Marketed as a federally legal alternative to ∆-9-THC, ∆-8-THC products have gained popularity post the 2018 Farm Bill.
– Delta-8 THC products are available in various forms and widely accessible in retail outlets.
– Challenges related to unregulated distribution, illicit activities, and enforcement of state laws have been observed in the market.
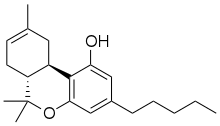
Δ-8-tetrahydrocannabinol (delta-8-THC, Δ8-THC) is a psychoactive cannabinoid found in the Cannabis plant. It is an isomer of delta-9-tetrahydrocannabinol (delta-9-THC, Δ9-THC), the compound commonly known as THC, with which it co-occurs in hemp; natural quantities of ∆8-THC found in hemp are low.
| |
| |
| Names | |
|---|---|
| IUPAC name
6,6,9-trimethyl-3-pentyl-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.165.076 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H30O2 | |
| Molar mass | 314.5 g/mol |
| Density | 1.0±0.1 g/cm3 |
| Boiling point | 383.5±42.0 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Psychoactive effects are similar to that of Δ9-THC, with central effects occurring by binding to cannabinoid receptors found in various regions of the brain. During the production process of converting cannabidiol extracted from hemp to ∆8-THC, toxic chemical reagents are used, which can contaminate the product. In the US, ∆8-THC products are neither mandatorily tested nor FDA approved, hence concern has been raised about their safety. In addition, as of 2022, the safety profile of regular, long-term delta-8-THC consumption is unknown.
Partial synthesis of ∆8-THC was published in 1941 by Roger Adams and colleagues at the University of Illinois. After the 2018 United States farm bill was signed, ∆8-THC products partially synthesized from compliant sources (including industrial hemp and derivative cannabidiol extracts) experienced a rise in popularity; THC products have been sold in licensed, regulated recreational cannabis and medical cannabis industries within the United States only in California, Pennsylvania, and regulated in Michigan and Oregon. According to a March 2024 study, 35% of US twelfth graders have used ∆8-THC over the past 12 months.





