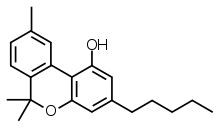
**Chemical Structure and Synthesis:**
– Cannabinol (CBN) is a classical cannabinoid derived from Cannabis Sativa L.
– CBN has a slower metabolic profile compared to THC due to an additional aromatic ring.
– CBN lacks double bond isomers and stereoisomers.
– CBN is generated through the oxidation of THC and can degrade into HU-345 from oxidation.
– CBN can be found in aged and stored cannabis.
– Orally administered CBN undergoes first-pass metabolism in the liver.
**Pharmacology and Receptor Targets:**
– CBN is a low affinity partial agonist at CB1 and CB2 receptors.
– CBN has antimicrobial effects in cell culture and acts as an ANKTM1 channel agonist at high concentrations.
– CBN inhibits GI motility in preclinical rodent studies.
– CBN activates CB1 and CB2 receptors, with a lower affinity for CB1 compared to THC.
– CB2 receptors, primarily associated with immune function, may interact with nociceptive and immune-related signaling.
**Pharmacokinetics and Metabolism:**
– A small study found a highly variable half-life of 32±17 hours upon intravenous administration.
– CBN is metabolized by CYP2C9 and CYP3A4 liver enzymes.
– The half-life of CBN is sensitive to genetic factors affecting the levels of these enzymes.
– Orally administered CBN has low bioavailability following oral administration.
**Legal Status in the United States and Internationally:**
– CBN is not listed in the schedules set out by the United Nations Single Convention on Narcotic Drugs.
– In the United States, extracts from Cannabis sativa L. are legal under federal law if THC concentration is 0.3% or less.
– Sales or possession of CBN could potentially be prosecuted under the Federal Analogue Act.
– The 2018 Farm Bill legalized extracts from Cannabis sativa L. with THC concentration of 0.3% or less.
– Legal and regulatory issues governing cannabis and cannabis-derived products are addressed in the U.S.
**Research, Medicinal Uses, and Future Potential:**
– Various studies and publications discuss cannabinol, its pharmacology, and therapeutic potential.
– Research explores the use of cannabis and cannabinoids for cancer treatment and other medical conditions.
– Cannabinol is being studied for its anti-inflammatory, neuroprotective, and potential sleep aid properties.
– The therapeutic potential of cannabis, cannabidiol (CBD), and cannabinoid-based pharmaceuticals is examined.
– Ongoing research aims to understand the endocannabinoid system and develop future therapeutic strategies.
Cannabinol (CBN) is a mildly psychoactive cannabinoid (e.g., CBD) that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS).
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.216.772 |
| Chemical and physical data | |
| Formula | C21H26O2 |
| Molar mass | 310.437 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 76–77 °C (169–171 °F) |
| Solubility in water | Insoluble in water, soluble in methanol and ethanol mg/mL (20 °C) |
| |
| |
| | |
In 1896 Cannabinol was first discovered in Cannabis by Thomas Barlow Wood, W.T Newton Spivey, and Thomas Easterfield. In the early 1930s CBNs structure was identified by Robert Sidney Cahn, marking the first development of a cannabis extract.
Its chemical synthesis were achieved by 1940, followed by some of the first basic research studies to determine the effects of individual cannabis-derived compounds in vivo. Although CBN shares the same mechanism of action as other phytocannabinoids (e.g., Delta-9-tetrahydrocannabinol, Δ9-THC), it has a lower affinity for CB1 receptors, meaning that much higher doses of CBN are required in order to experience effects, such as mild sedation.





